Carbohydrates: A good energy source
What is Carbohydrates?
- Carbohydrates = also called saccharides (sugars).
- They are the most common biological molecules.
- Made of C, H, and O in ratio 1: 2: 1 → (CH₂O)n. (where minimum n=3 or more)
Definition: They are aldehyde or ketone derivatives of polyhydroxy alcohols (means they contain many –OH groups along with aldehyde/ketone).
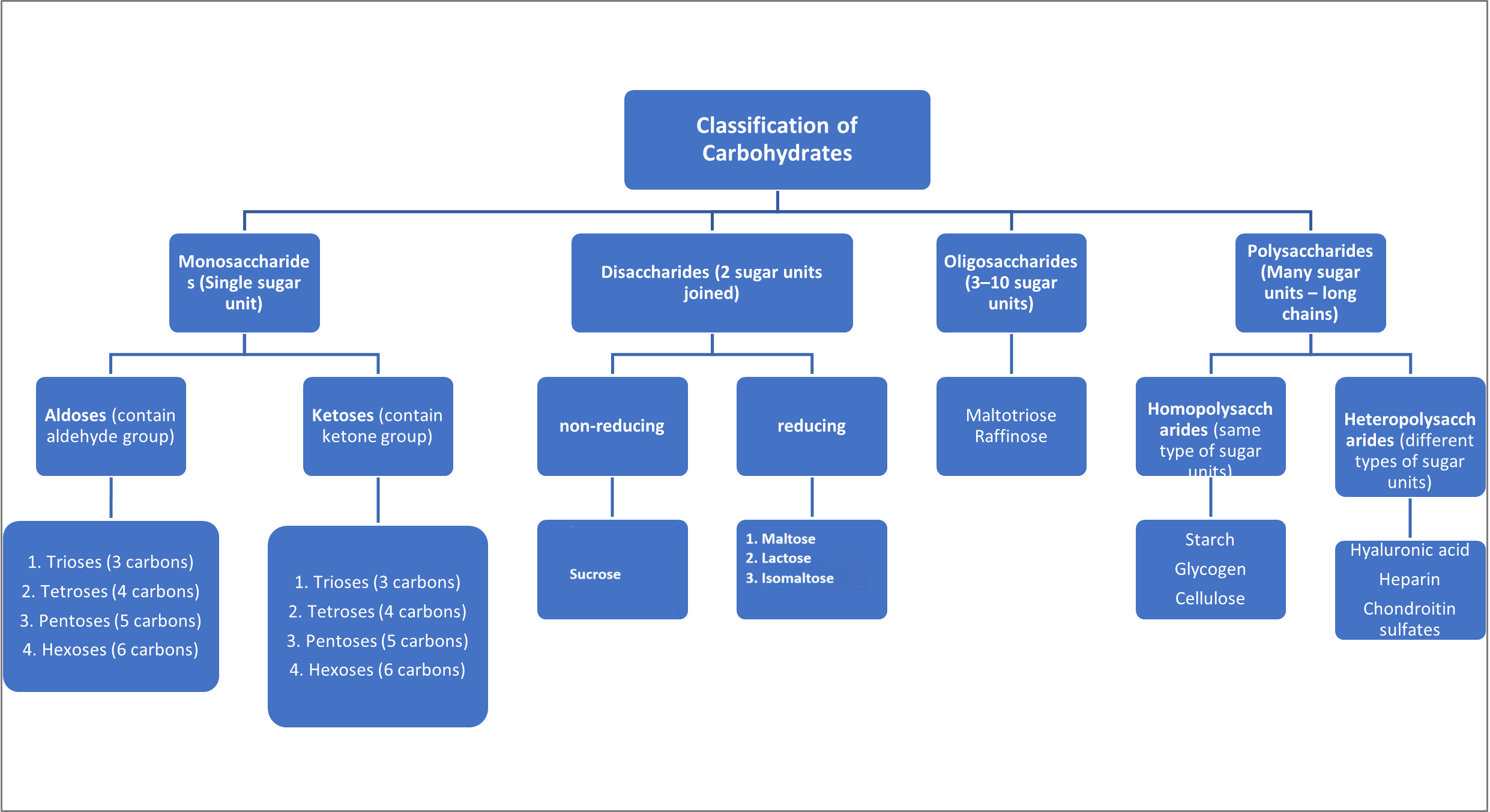
Classification of Carbohydrates
Quick Tip-
- Mono = single unit
- Di = double unit
- Oligo = few (3–10 units)
- Poly = many units
MONOSACCHARIDES-
- Definition: Monosaccharides are the simplest carbohydrates.
- They cannot be broken down further.
- They are aldehyde or ketone derivatives of straight-chain polyhydroxy alcohols (with 3 or more carbons).
Classification of Monosaccharides
- Based on carbonyl group
- Aldoses → contain aldehyde (–CHO) group
- Example: Glucose
- Ketoses → contain ketone (–CO) group
- Example: Fructose
Ketose names are made by adding -ul before “-ose” (e.g. erythrose → erythulose).
- Based on number of carbons
| Type | No. of Carbons | Examples (Aldoses) | Examples (Ketoses) |
| Trioses | 3 | Glyceraldehyde | Dihydroxyacetone |
| Tetroses | 4 | Erythrose | Erythrulose |
| Pentoses | 5 | Ribose | Ribulose |
| Hexoses | 6 | Glucose | Fructose |
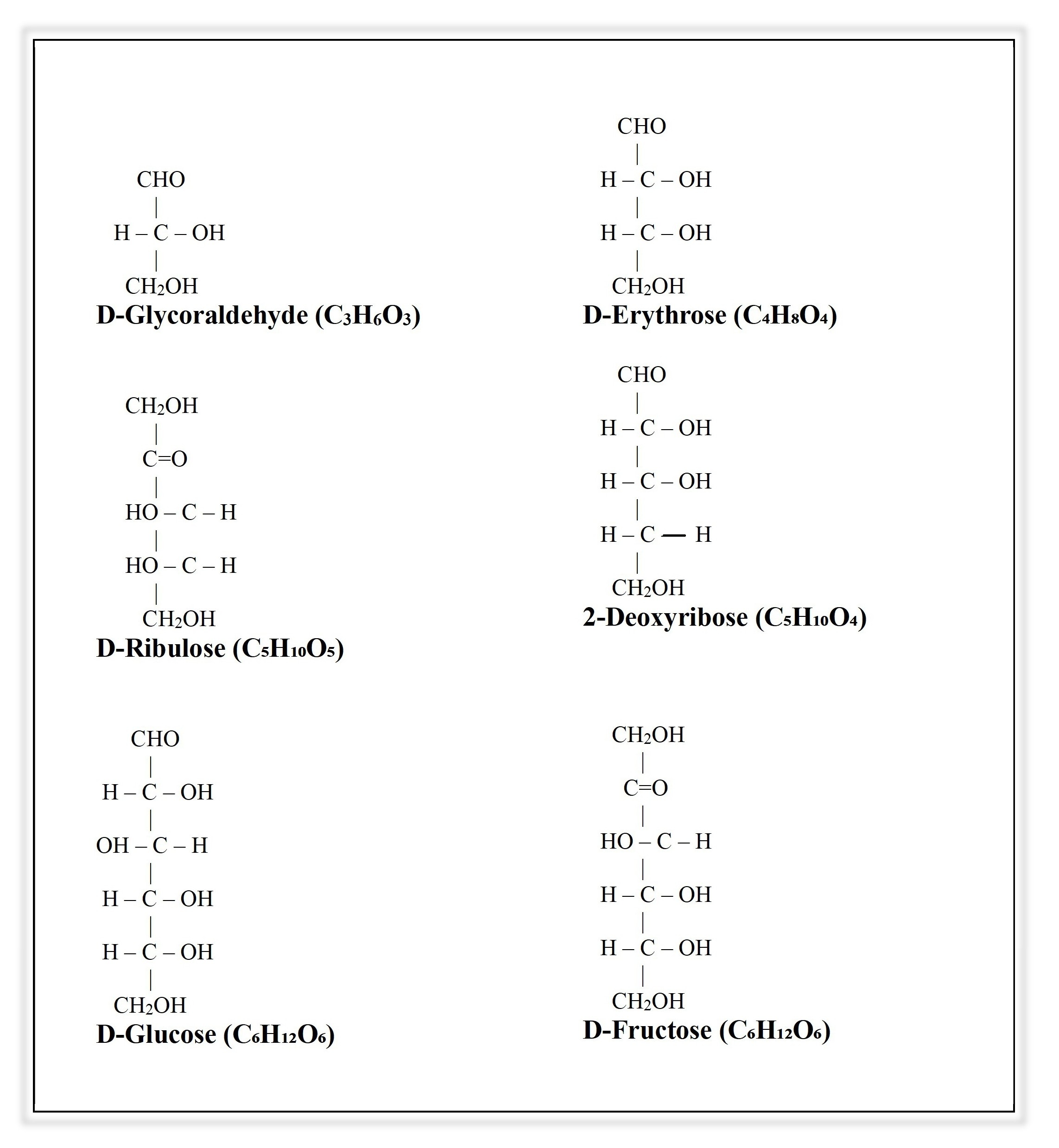
- Trioses (3C)
- Smallest monosaccharides.
- Examples:
- Glyceraldehyde (aldotriose)
- Dihydroxyacetone (ketotriose)
- Examples:
- Important in glycolysis as intermediates (Glyceraldehyde-3-phosphate, DHAP).
- Tetroses (4C)
- Examples:
- Erythrose (aldotetrose)
- Erythrulose (ketotetrose)
- Important in HMP shunt pathway.
- Pentoses (5C)
- Examples:
- Ribose (aldopentose) → in RNA
- Xylose (aldopentose)
- Ribulose (ketopentose) → intermediate in HMP shunt.
- Hexoses (6C)
- Most important sugars in human body.
- Examples:
- Glucose (aldohexose) → main energy source (blood sugar).
- Galactose (aldohexose) → part of lactose (milk sugar).
- Fructose (ketohexose) → part of sucrose (table sugar).
Quick Revision-
- 3C = triose (glyceraldehyde, dihydroxyacetone)
- 4C = tetrose (erythrose, erythrulose)
- 5C = pentose (ribose, ribulose)
- 6C = hexose (glucose, galactose, fructose)
Derived Sugars/carbohydrates-
These are sugars or carbohydrates that are modified forms of monosaccharides:
- Acid Sugars–
- When the aldehyde group (C1 of aldose) is oxidized → forms aldonic acid
- Example: Gluconic acid
- When the alcohol group at C6 is oxidized – forms uronic acid
- Example: Glucuronic acid
CHO
|
H – C – OH
|
OH – C – H
|
H – C – OH
|
H – C – OH
|
COOH
- Sugar Alcohols–
- When sugars are reduced – they form polyhydroxy alcohols (alditols).
- Examples:
- Ribitol – found in FMN & FAD (coenzymes)
- Xylitol – used as sweetener (in gums, candies)
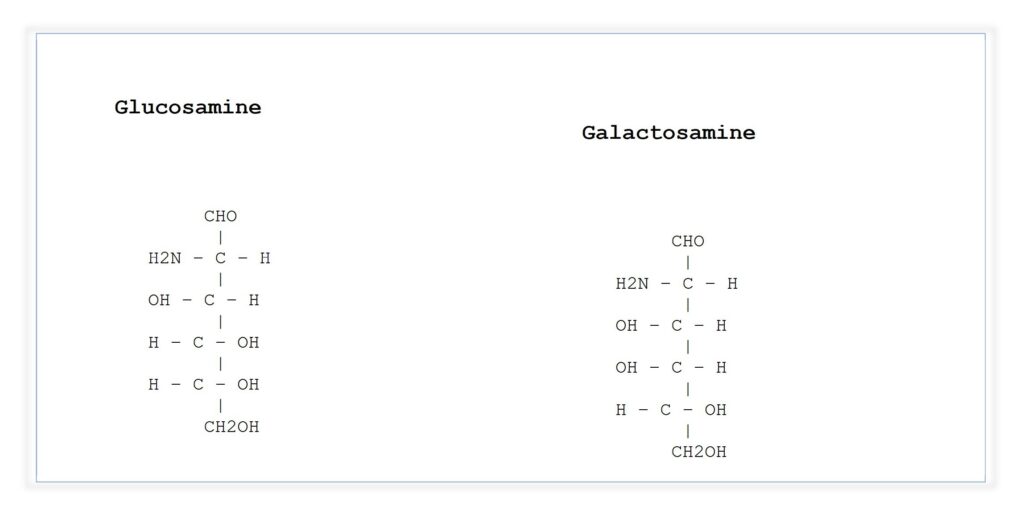
- Amino Sugars–
- Formed when the –OH group at carbon-2 is replaced by –NH₂.
- Examples: Glucosamine, Galactosamine.
- Some exist as acetylated forms → N-acetyl derivatives.
- Found in:
- Glycosaminoglycans (hyaluronic acid, chondroitin sulfate)
- Antibiotics (erythromycin)
- N-acetylneuraminic acid (sialic acid) → important in glycoproteins & glycolipids.
- Glycosides–
- When carbon-1 of a sugar or carbohydrates reacts with an alcohol like methanol→ forms glycosidic bond (a type of bond that connect a carbohydrate to another group).
- Example: Methylglycoside (C7H14O6).
- Found in glycosaminoglycans.
Disaccharides
Disaccharides have 2 monosaccharides joined by a glycosidic bond.
Examples: Maltose, Lactose, Sucrose (biologically important).
Maltose
- Made of: 2 α-D-glucose units.
- Linkage: α(1→4) glycosidic bond.
- Leaves one free –OH group at carbon-1 → reducing sugar.
- Formed by hydrolysis of starch & glycogen.
- Broken down by enzyme maltase → gives 2 glucose units.
Lactose
- Made of: β-D-galactose + β-D-glucose.
- Linkage: β(1→4) glycosidic bond.
- Found in milk.
- Reducing sugar (because glucose unit has free aldehyde or ketone group).
Lactose
- Also known as milk sugar.
- Structure: β-D-galactose + β-D-glucose
- Linkage: β(1→4) glycosidic bond
- Found in: milk (major sugar in mammalian milk)
- Properties:
- Reducing sugar/ carbohydrates (because glucose part has free aldehyde or ketone group).
- Hydrolyzed by enzyme lactase (β-D-galactosidase) → produces glucose + galactose.
| Disaccharide | Components | Linkage | Reducing? | Found in |
| Maltose | Glucose + Glucose | α(1→4) | Yes | From starch digestion |
| Lactose | Galactose + Glucose | β(1→4) | Yes | Milk |
| Sucrose | Glucose + Fructose | α(1→2)β | No | Sugarcane, fruits |
Oligosaccharides
- Oligosaccharides made of 3–10 monosaccharide units linked by glycosidic bonds.
- Examples: Maltotriose, Raffinose, etc.
- Most are not digested in humans.
- Many are O-linked to proteins (glycoproteins, mucins).
- Functions:
- Structural role in proteins.
- Protein glycosylation (adding sugar chains to proteins) → affects stability, activity.
Polysaccharides
- Large polymers of more than 10 monosaccharides.
- Linked by glycosidic bonds.
- Properties:
- Not sweet
- Not reducing
- Sparingly soluble in water
Types:
- Homopolysaccharides → made of only one type of sugar
Examples: Starch, Glycogen, Cellulose (all polymers of glucose)
- Heteropolysaccharides → made of different sugars (covered later, e.g. hyaluronic acid, heparin).
